JOULE'S
LAW
THE HEATING EFFECT OF AN ELECTRIC CURRENT
MOTIVATION
I shall lose no time in repeating and extending these experiments, being satisfied that the grand agents of nature are, by the Creator's fiat, indestructible; and that whatever mechanical force is expended, an exact equivalent heat is always obtained.
JOULE, 1843
For a considerable period in the history of physics heat was thought to be some material, but "imponderable," fluid which flowed between hot and cold bodies. The unit of heat (either calorie or British thermal unit) was defined in terms of the rise in temperature produced when heat flowed into unit mass of water.
At the same time in history other workers were concerned with a study of the relationships existing between work done on a system and the mechanical energies of the system. The units of mechanical energy (foot-pound and newton-meter or joule) were naturally defined in terms of the work concept. Even later scientists studying the phenomena associated with electricity elected to use the mechanical units for expressing electrical energy (joules) and power (watts or joules per second).
With the work of Joule (see above) and others, it became apparent that heat was another manifestation of energy and could properly be measured in mechanical units (joules) provided the relationship between those units and the previously established thermal units (calories) could be determined. We write:
![]() (1)
(1)
where J is called the mechanical equivalent of heat and is expressed in joules per calorie.
In this experiment you are asked to use an electrical calorimeter to determine the numerical value of J. The electrical energy supplied to the calorimeter is given by
![]() (2)
(2)
where V is the potential difference, I is the current and t is the time.
Since no change of state occurs, we can write the heat energy produced in the calorimeter as
![]() (3)
(3)
where m represents mass, c the specific heat and D T the temperature change. The summation indicates that you are to write a term, mc D T, for each object which undergoes a temperature change as a result of the electrical energy supplied. Since the assumption is that all electrical energy is converted to heat in an insulated vessel, we should be able to use Equation 1 to determine a value for J.
SPECIFIC OBJECTIVES:
When you have completed this experimental activity you should be able to: (1) construct an electrical circuit from the schematic diagram provided; (2) determine the electrical energy supplied to a system from measured values of V, I, R, t, etc; (3) conduct an experiment to measure the mechanical equivalent of heat; (4) relate the joule or calorie of energy to the kWh unit of electrical energy; and (5) understand the term "water equivalent."
EXPERIMENTAL ACTIVITY:
Prepare a data sheet adequate to record values of V, I, and Temperature as a function of time. You will be making readings at two-minute intervals for about 60 minutes.
Place the thermometer in the stopper so that the bulb will be submerged. A little stopcock lubricant will make it go in easily.
Record in your notebook such data as may be required to determine the heat energy produced in the calorimeter. Think carefully about what you will need to know. The specific heat capacity of the calorimeter and stirrer is 0.215 cal/gm-oC and the water equivalent of the heater element is 3.65 grams.
Use enough cold water from the hall fountain to completely submerge the heating element. The water should be colder than room temperature by 10oC or more. How will you determine how much water you have?
Connect the circuit a shown in the diagram. Do not plug the power supply into the outlet until your circuit has been approved by your instructor.
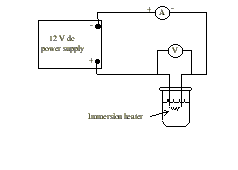
Figure 1
ABOVE
ALL -- DO NOT TURN ON THE CURRENT UNLESS THE HEATER ELEMENT IS IN WATER.
When all is ready, record the temperature at two-minute intervals for a period of four minutes before turning on the heater. Make all temperature readings as precise as possible -- typically you should be able to read to tenths of a degree if you are careful.
At t=4 minutes, turn on the power supply and immediately adjust the current to 2.0 amperes. You must keep the current at this constant value throughout the experiment. Continue to record the voltage, current, and temperature at two minute intervals until the temperature rises at least 20oC above the initial temperature. Continue reading the temperature for a period of six to eight minutes after the heating current is turned off.
ANALYSIS OF RESULTS
Plot a graph of temperature as a function of time; include the periods before and after the heating cycle. Explain any non-linear behavior you observe. Was it necessary to make any adjustments during the heating cycle to keep the current constant? If so, explain why.
Calculate the heat energy produced in the calorimeter. Show your work in a neat, well-organized manner. Calculate the magnitude of J and compare with the standard value of 4.19 joule/cal. What is your percent error?
QUESTIONS:
- Was there any evident of heat flow into or out of the
calorimeter during the pre- or post-heating periods? If so, what was the
evidence and how might this affect the results of your experiment? Be
specific.
- What is meant by the term "water equivalent"?
- Why were you cautioned not to turn on the current until
the coil was submerged in water?
- How many joules of energy have been supplied if your
electric meter records one kilowatt-hour? Show your calculation.
FINAL SUMMARY:
In your report summary, include the value you found for J, and indicate what you judge to be the most important source of error in this experiment. What conclusions did you draw about the relationship of thermal units and mechanical energy units?